Drug Stability Test Chamber
2022-04-23 19:58:43

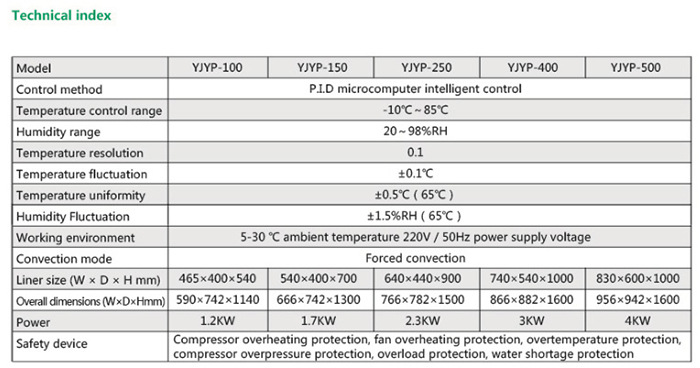
Product Usage
The drug stability test chamber is used for the intensive research in the pharmaceutical industry, medicine, biotechnology, food industry, the electronics industry and all related industries including life sciences. The requirements of the WHO principles are long-term stability test conditions and 25 C / 60% RHhumidity. In the accelerated test, the humidity of 40 °C / 75% RH is tested for 6 months. It is a field of stability test systems for the pharmaceutical industry. It mainly simulates temperature and humidity tests in environmental climate.
Information recommendation







